Fostering collaborative approaches to qualitative data analysis and building capacity among our African colleagues
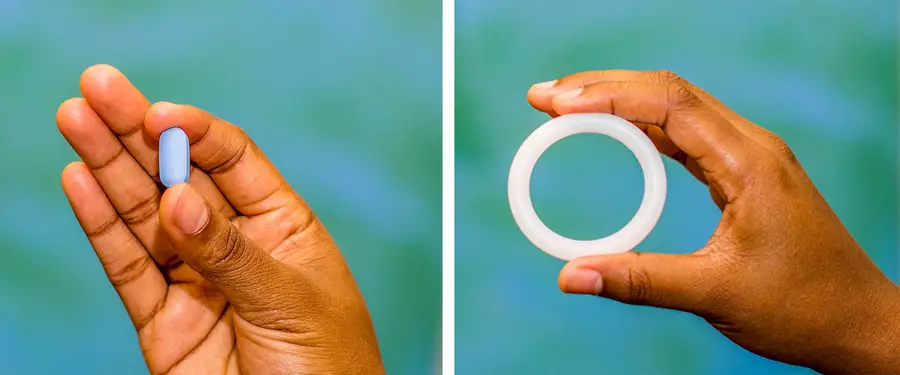
The Microbicide Trials Network (MTN) is an NIH-funded worldwide collaborative clinical trials network focused on the evaluation of products for preventing the sexual transmission of HIV. Based at the University of Pittsburgh’s Magee-Women’s Research Institute, many of its studies take place in sub-Saharan Africa, where women are at especially high risk of HIV. MTN-041, or the MAMMA Study (Microbicide/PrEP Acceptability among Mothers and Male Partners in Africa), was a recently completed qualitative study that explored community attitudes and perceptions about the use of two HIV prevention products—daily oral Pre-Exposure Prophylaxis (PrEP) and a monthly vaginal ring—during pregnancy and breastfeeding. The study was designed to inform MTN’s implementation of two planned clinical trials of these products in pregnant and breastfeeding women.
MTN-041 study data were collected by clinical research sites that are part of NIH-funded clinical trial units within local universities, and many of which have affiliations with U.S. universities. Clinical research staff are rarely part of the full lifecycle of the data, especially through the analysis and publication process. As a result, their valuable contribution is not sufficiently accounted for once data collection is completed. At a meeting of MTN study teams in 2018, we organized a special workshop that allowed research staff members an opportunity to participate in a rapid preliminary analysis of the data they collected earlier in the year.
The MTN-041/MAMMA study was conducted in four countries in sub-Saharan Africa: Malawi, South Africa, Uganda, and Zimbabwe. Our workshop took place in September 2018 in Cape Town. The 26 attendees included qualitative interviewers, community representatives and investigators of record from each site. Many of these individuals were attending the MTN Regional Meeting for the first time; while it is typical for Investigators of Record and other research staff to attend the meeting, qualitative staff do not regularly have this opportunity.
In the weeks leading up to the workshop, each participant was asked to read one transcript from a focus group discussion (FGD) with pregnant and breastfeeding women that took place at their site, and to come prepared to discuss their perspectives. While this workshop focused on initial analysis of data from FGDs with pregnant and breastfeeding women, it is worth noting that in the MAMMA study, FGDs were also conducted with male partners and mothers/mothers-in-law of pregnant and breastfeeding women.
When everyone came together for the workshop, and reported on the transcript they had reviewed, the group identified eight topic areas:
- Health practices (nutrition, hygiene practices, sexual practices)
- HIV risk (male partner promiscuity, condom use)
- Perceived facilitators and barriers to oral PrEP use
- Perceived facilitators and barriers to vaginal ring use
- Recommendations for future trials of these products that will involve pregnant and breastfeeding populations (e.g., MTN-042; MTN-043)
- Key influencers (e.g., male partners, friends, family, health workers, traditional birth attendants, religious leaders)
- Prohibited and acceptable practices related to pregnancy and breastfeeding
- Parking lot (i.e., themes that could not be readily categorized under the other topic areas).
Attendees broke into teams with representation from each study site. Each group had a facilitator (staff member from RTI International or FHI 360) to drive discussion about identified themes. Groups rotated around to each of the eight topic boards mentioned above. At each station, they used spray glue to paste and re-arrange theme note cards into proximity clusters on the topic boards.

The next day, new teams were formed, again mixed across sites. Each group was assigned to one topic board, where they sorted the notecards and grouped them into subthemes (e.g., ‘male partner promiscuity’ as a sub-theme of HIV risk). Everyone then came together to hear what each group had identified as the key themes and subthemes from their topic area, and to organize these into a preliminary code book.
In a post-meeting electronic survey circulated to the workshop attendees, all respondents indicated that they found the workshop to be fun, engaging and beneficial for building skills in qualitative analysis and theme generation. Many participants stated that they felt valued to be part of the analysis stage of the project.
Since I participated in data collection, I felt valued being part of the analysis workshop, since we have the first hand information and were able to clarify gaps. — Participant from Uganda
The findings generated during the workshop directly informed the development of the MTN-041 codebook, which in turn was used to systematically analyze all study data. The behavioral data from MTN-041 will directly inform the implementation of two large clinical trials in the coming months that will investigate the safety and pharmacokinetics of oral PrEP and vaginal ring use during pregnancy and breastfeeding (MTN-042; MTN -043).
Our team at RTI will strive to hold similar workshops for other projects in the future. Through engaging and creative experiences like these, all team members gain perspective on what happens to the data after they are collected and before they are published. Enough cannot be said about the importance of site staff being part of this process; it facilitates cross-country dialogue and idea generation and improves the interpretation of our qualitative data, as well as resulting in a greater appreciation for the research process itself.
- Microbicide Trials Network (MTN)
- Wits Reproductive Health and HIV Institute
- Makerere University – Johns Hopkins University Research Collaboration
- Johns Hopkins Project-College of Medicine, University of Malawi
- University of Zimbabwe College of Health Sciences Clinical Trials Research Centre



