Introduction
Colorectal cancer (CRC) is the second-most expensive cancer to treat, at an estimated projected annual cost in the United States of $19 to $26 billion in 2020 (Mariotto et al., 2011). The high costs and substantial cost-sharing associated with cancer medicines may put Medicare beneficiaries treated for CRC at risk for delaying or discontinuing their therapies, possibly increasing other health care use, such as inpatient admissions or emergency department (ED) visits (Hsu et al., 2008; Tamblyn et al., 2001).
In January 2006, with US drug prices continuing to climb ever higher, the Centers for Medicare and Medicaid Services implemented the optional Medicare prescription drug benefit (Part D) program to offer subsidized drug plans to all Medicare beneficiaries (Lichtenberg & Sun, 2007). Before Part D, Medicare beneficiaries with cancer faced substantial out-of-pocket (OOP) costs: according to data from the Medical Expenditure Panel Survey, from 1996 to 2005, 57 percent of Medicare beneficiaries over 65 years of age with cancer had annual OOP costs exceeding 10 percent of their annual income (Lines, 2008a, 2008b). More-recent research suggests that since Part D’s introduction, average OOP burden among Medicare beneficiaries with cancer has declined by 43% (Kircher et al., 2014a).
Published data from the National Cancer Institute’s (NCI’s) Surveillance, Epidemiology, and End Results (SEER)-Medicare program indicate that the proportion of Medicare fee-for-service (FFS) beneficiaries with CRC who were covered by the Part D program during the calendar year after diagnosis ranged from 55 percent in 2007 to 71 percent in 2013; about 57 percent to 70 percent of Medicare FFS beneficiaries without cancer were enrolled in Part D during the same period (National Cancer Institute, 2016). Those who elect not to enroll in Part D initially can avoid paying a late enrollment penalty for subsequent Part D enrollment by maintaining “creditable coverage” for prescription drugs (meaning coverage from another source, such as a current or former employer or union, that is expected to pay at least as much as the standard Medicare prescription drug coverage, on average) (Medicare.gov, 2018).
To date, few researchers have studied the impact of Part D coverage on costs or outcomes among people with cancer (Blanchard et al., 2013; Hsu et al., 2008; Medicare Today, 2016). Research not specific to people with cancer has found that Part D has reduced OOP costs (Lichtenberg & Sun, 2007) and mortality (Kaestner et al., 2014; Semilla et al., 2015); decreased hospital admissions, overall resource use, and total Medicare costs (Kaestner & Khan, 2012; Kaestner et al., 2014); and increased use of prescription medicines (Kaestner & Khan, 2012; Lichtenberg & Sun, 2007; Liu et al., 2011)—all while keeping monthly premiums and program costs below projections (Hoadley, 2012) and receiving high patient experience ratings (Medicare Today, 2016).
The objective of this study was to evaluate patterns of Part D uptake and costs and to understand the effects of coverage on inpatient stays and ED use among a cohort of individuals diagnosed with CRC in the United States from 2007 through 2010 and matched beneficiaries without cancer. We evaluated the following research questions: (1) What proportion of beneficiaries with and without CRC had Part D coverage during this time period, and how did this change over time? (2) How did Part D expenditures compare between Medicare beneficiaries with and without CRC? (3) Among people with CRC, was Part D coverage associated with any differences in the occurrence or volume of inpatient admissions or ED use during the initial treatment phase?
Methods
We used the most-recent data available at the time of our study, which covers individuals diagnosed from January 2007 through December 2010 and followed through December 2013. We used cancer registry data from the SEER database linked with Medicare claims and enrollment data (SEER-Medicare). SEER-Medicare data have been used in more than 1,600 peer-reviewed studies since 1993 (National Cancer Institute, 2017). The data also include cancer stage, which is difficult to infer from claims data alone, and which is often a major driver of costs and clinical outcomes (Chawla et al., 2014).
Sample Selection and Cohort Matching
We included continuously enrolled Medicare FFS beneficiaries aged 65 years and older at baseline who resided in a SEER area and had at least 36 months of follow-up. Note that although Part D plans are also available to Medicare Advantage (MA) enrollees, SEER-Medicare data only include FFS beneficiaries. The cancer cohort included individuals who were diagnosed with incident CRC (defined using International Classification of Diseases for Oncology, Third Edition, site codes C180–C189, C199, C209, and C260). Although the Part D program began in 2006, reliable Part D data reporting in the SEER-Medicare data set began in 2007. Thus, we included only individuals diagnosed from January 2007 through December 2010 to ensure at least 36 full months of follow-up. The maximum follow-up time was 95 months.
For both the cancer cohort and matched no-cancer cohort, we included those who were continuously enrolled in FFS Medicare Parts A and B for the entire study period, including at least 12 months before their diagnosis date (or index date, among people without CRC). We excluded individuals with dual (Medicare and Medicaid) coverage because they are automatically enrolled in Part D and so could not provide differential insights into enrollment trends.
We constructed a matched comparison cohort from a 5 percent random sample of Medicare beneficiaries who had no known diagnosis of cancer and resided in SEER areas. We matched at a rate of 1:1 on sex (M, F), race (white, black, other), SEER registry area, age category (65–69, 70–74, 75–79, 80–84, and 85 or older), and NCI Comorbidity Index category (0, 1, or 2+), calculated based on inpatient and physician claims during the 12-month pre-index period using programs supplied by NCI (Klabunde et al., 2000). The NCI Comorbidity Index was developed specifically to analyze claims for cancer patients and excludes cancer-related morbidity (National Cancer Institute, 2019). Comorbidity is a common matching variable in utilization studies (Hanchate et al., 2010; Snyder et al., 2009). In this study, including comorbidity was important; otherwise, it might have confounded the association between complete Part D coverage (exposure) and different kinds of utilization (outcome measures). Each individual was matched to only one other individual, and people with cancer who could not be matched (n = 72 of 13,151 who met inclusion criteria) were dropped. The matched comparison person was assigned an index date corresponding to their match’s CRC diagnosis date, and henceforth, we refer to this as the index date for both cohorts. After matching, we examined the balance between cohorts on sociodemographic and clinical characteristics.
Measures
We defined the exposure variable, “complete Part D coverage,” as 12 months of coverage in the diagnosis year and each of the 2 subsequent calendar years. Because Part D enrollment is strongly associated with calendar years (rather than 12-month postindex periods), we examined trends by calendar year. The number of individuals with partial-year coverage was too small to analyze separately (less than 3 percent of the cancer cohort) and showed no clear patterns (i.e., there were no clear peaks in the distribution of the number of months of coverage among those with less than 12 months). Therefore, we divided each cohort into those with complete Part D coverage and all others. Individuals in the partial/no Part D groups had some Part D expenditures in the data, but we do not know what proportion of total drug spending the observed amount represents for those individuals. As a robustness check, we also conducted an analysis that excluded the partially enrolled individuals.
Our outcome measures included total Part D costs, any hospitalization, the number of hospitalizations, any ED visit, and number of ED visits during the 12 months post-index, generally considered the active treatment phase for CRC patients (Lang et al., 2008). We adjusted all Part D costs to constant (2012) US dollars using the gross domestic product deflator (U.S. Bureau of Economic Analysis, 2017).
Statistical Analysis
We analyzed sociodemographic and clinical characteristics by cohort (CRC compared with no cancer) and group (complete Part D compared with others), using Pearson chi-squared tests to identify significant differences. We examined the proportion of people who had complete Part D coverage overall and in their pre-index year, index year, post-index year 1, and post-index year 2, separating our analysis by index year (2007 through 2010), and we tested for differences between cohorts in enrollment rates. We also tested for any trends in uptake over time using Kendall's tau (continuity corrected). Because people indexed in 2007 could not have had a pre-index year (because the program was not reporting useable data in 2006), the descriptive analysis of pre-index coverage excludes individuals indexed in 2007.
To estimate the adjusted effects of Part D on the number of hospitalizations and ED visits in the 12-month post-index period, we first counted inpatient hospitalizations and outpatient ED visits. We modeled differences between those with and without complete Part D separately for the CRC and no-cancer cohorts. We investigated both zero-inflated and noninflated models and chose the model that was statistically preferable on the basis of likelihood ratio tests. Specifically, in the CRC cohort, we used a regular multivariable Poisson regression model; in the no-cancer cohort, we used zero-inflated Poisson to account for the large proportion of people with no hospitalizations (92 percent). To estimate the adjusted effects of complete Part D enrollment on ED use, we used negative binomial models; according to the Vuong tests, zero-inflated models were not statistically preferable. To estimate the effects of complete Part D coverage on any hospitalization or ED visit, we used logistic regression models.
Models in the CRC cohort controlled for site (rectal vs. colon), Stage 3 or 4 (vs. 1, 2, or unstaged), comorbidity, US Census region, rural residence, female sex, age group (65–74 [reference group], 75–84, or 85+ years), race or ethnicity (indicators for black, Hispanic, or other race or ethnicity), marital status, residence in a high-poverty US Census tract (> 20 percent of households with incomes under the Federal poverty level), and diagnosis year. Models in the no-cancer cohort controlled for all the same covariates except site and stage (not applicable), marital status (not available), and neighborhood poverty (not available). We report beta coefficients, standard deviations (SDs), and P-values for the results of the negative binomial models. We report adjusted odds ratios and 95% confidence limits for the results of the logistic models. All tests were two-tailed. We defined statistical significance as P < .05 and conducted analyses in StataMP v.14.2 (StataCorp LP, College Station, Texas).
Results
The cohort of Medicare FFS beneficiaries with CRC included 12,774 people, of whom 39 percent had complete Part D coverage (Table 1).
Table 1.
42549Sociodemographic and clinical characteristics by Part D enrollment among Medicare fee-for-service beneficiaries with and without colorectal cancer
N/A: not applicable/not available
a Complete Part D coverage is defined as continuous Part D coverage in the index year and 2 subsequent years.
b Excludes 16 individuals with unknown state of residence or residence in Puerto Rico/Virgin Islands
The matched comparison cohort included 12,774 Medicare FFS beneficiaries without cancer, of whom 38 percent had complete Part D coverage (P for difference between cohorts = .119). Within both cohorts, individuals with Part D coverage differed significantly from those without complete coverage on sex, race, rurality, US Census region, and comorbidity category. Among beneficiaries with CRC, those with complete Part D coverage had a significantly different age distribution (with more enrollees in the 65–74 age category) and were less likely to be married. Among both cohorts, those with complete Part D coverage were more likely to be in the youngest age group (65–74 years), female, and white; to live in a rural location; and to live in the Midwest.
In Figure 1, we show trends in Part D uptake by cohort and time (relative to index).
Figure 1.
42552Patterns of Part D enrollment among Medicare FFS beneficiaries with and without colorectal cancer
a Individuals indexed/diagnosed 2008–2011.
b Individuals indexed/diagnosed 2007–2011.
Notes: Shown are average annual Part D coverage rates among individuals diagnosed with colorectal cancer (CRC) and matched comparison beneficiaries in the first years of the Part D program (2007–2011). Although people with CRC had slightly higher coverage rates than those without CRC across all years, we found a significant (P = 0.003) difference in cohort coverage rates only in the first year after diagnosis.
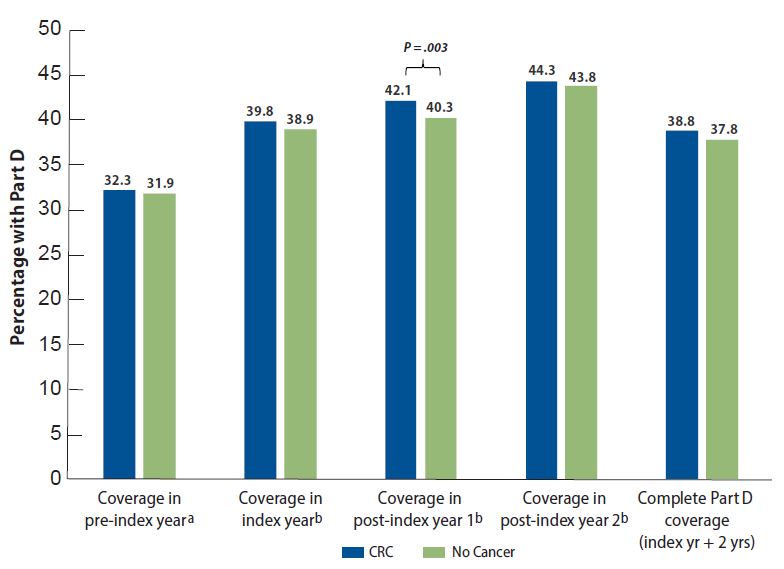
Comparing the CRC and matched cohorts, individuals with CRC were significantly more likely to be covered by Part D in the year after being indexed or diagnosed (P = .003), but no other times showed significant differences between cohorts. Both cohorts showed significantly increased rates of Part D uptake over time (CRC: P = .004; no cancer: P = .002).
Total 12-month Medicare Part D prescription drug costs are provided in Table 2.
Table 2.
42550Observed prescription drug costs and healthcare utilization by Part D enrollment among Medicare fee-for-service beneficiaries with and without colorectal cancer
Est. = estimate; SE = standard error.
a Complete Part D coverage defined as continuous Part D coverage in the index year and 2 subsequent years.
Among those with complete Part D coverage, costs did not significantly differ between people with and without CRC; both groups had costs of around $3,100, as opposed to around $100 in the no/partial Part D groups. The CRC cohort had substantially higher ED and hospitalization rates per 1,000 beneficiaries than the no-cancer cohort. The hospitalization rate was nearly 10 times higher, whereas the ED rate was about 5 times higher among those with CRC. Neither cohort showed any significant difference in hospitalization or ED visit rates between those with and without Part D coverage.
Within the CRC cohort, lack of Part D coverage was associated with no increase to slightly higher odds of an ED visit in the 12-month postindex period (adjusted odds ratio [aOR] = 1.09; 95% confidence interval = 1.00–1.18). The mean number of ED visits was 0.11 higher (P = .001) among those without Part D coverage (Table 3).
Table 3.
42551Adjusted effects of no/partial Part D among Medicare fee-for-service beneficiaries with and without colorectal cancer
LCL = lower confidence limit; aOR = adjusted odds ratio; SD = standard deviation; UCL = upper confidence limit
Note: Models adjusted the CRC cohort for site (rectal vs. colon), Stage 3 or 4 (vs. 1, 2, or unstaged), comorbidity, US Census region, rural residence, female sex, age group (65–74 [reference group], 75–84, or 85+ years), race or ethnicity (indicators for black, Hispanic, or other race or ethnicity), marital status, residence in a high-poverty US Census tract (> 20% of households with incomes under the Federal poverty level), and diagnosis year. Models in the no-cancer cohort controlled for the same covariates except site and stage (not applicable), as well as marital status and poor neighborhood (not available).
Within the no-cancer cohort, Part D enrollment showed no significant effect on ED use. After controlling for potential confounding factors, we found no significant effects of Part D enrollment on inpatient use within either cohort. In sensitivity analyses that excluded those with partial coverage, results were nearly identical (not shown).
Discussion
Continuous Part D enrollment during the diagnosis year and 2 subsequent years, termed “complete Part D coverage” in this study, was associated with a reduction in ED visits among nondual Medicare FFS beneficiaries with CRC over 65 years of age during the 12 months postindex, after adjusting for potential confounding effects. Among people with complete Part D coverage, no significant differences were observed between beneficiaries with CRC and those without cancer in Part D costs. Although Part D uptake significantly increased in the year postindex, no significant differences were observed between cohorts in overall uptake of the program. Of note, our estimates of Part D uptake differ from those published by the SEER-Medicare program (National Cancer Institute, 2016) because we excluded dual enrollees from our sample, as they are automatically enrolled in Part D (Lines et al., 2009).
Both CRC and non-CRC cohorts showed statistically significant increases in Part D uptake over time, consistent with trends in the larger Medicare population. According to the Kaiser Family Foundation, the program had average annual increases of 6.5 percent from 2006 through 2013 (Hoadley et al., 2014). Our study found that Part D enrollment increased by 9 percent over time in this sample of people with CRC and by 10 percent in the matched cohort. The differences we observe could be because we have only nondual FFS beneficiaries in our data.
The association of Part D with slightly reduced odds of an ED visit only among people with CRC is puzzling, partly because the Part D program does not directly cover cancer treatments for this population. Although oral chemotherapy for CRC was available during the study period in the form of capecitabine (Xeloda), it was covered under Part B, and not Part D, because it is an alternative form of the infusion therapy 5-Fluorouracil (D’Amato, n.d.). The rules for oral antinausea drugs are much the same (American Cancer Society, n.d.).
Because of these coverage policies, the observed effects of Part D among people with CRC could be related to better management of medication-sensitive conditions, such as diabetes, congestive heart failure, stroke, and myocardial infarction. As shown in Table 1, those with complete Part D coverage were significantly more likely to have at least one major comorbidity. Theoretically and empirically, improved access to medicines leads to improved adherence, which helps improve blood pressure, cholesterol, and blood glucose levels, potentially preventing or delaying ED visits (Blanchard et al., 2013; Brown & Bussell, 2011; Jha et al., 2012; Reynolds et al., 2016). Inpatient use in one period decreases overall ED use the next, suggesting that the intensive care provided during hospital stays may be effective at addressing clinical issues that could subsequently manifest in emergencies (Lines et al., 2017). Therefore, it is also possible that the effects among Part D enrollees in the CRC cohort are related to receiving more health care services in general.
Other research on the effects of Part D on outcomes among people with cancer is limited. One study used Medical Expenditure Panel Survey data for 2002 through 2010 and employed a difference-in-differences design to explore the effects of Part D enrollment on OOP costs, medicine use, hospitalizations, ED visits, and outpatient visits among Medicare beneficiaries with cancer (n = 4,729). The authors found that Part D was associated with a 43 percent decline in OOP costs and fewer outpatient visits (Kircher et al., 2014b). Studies among women with breast cancer have found that Part D was associated with reduced mortality, improved adherence to medicine, and reduced racial/ethnic disparities (Biggers et al., 2016; Nattinger et al., 2017). More broadly, researchers have failed to find any associations between Part D enrollment and self-reported health status, limitations in activities of daily living and instrumental activities of daily living, ED visits and hospital admissions (prevalence, counts, and spending), or death among Medicare enrollees, according to nationally representative data from the Medicare Current Beneficiary Survey (n = 56,293) combined with Medicare claims (Briesacher et al., 2015). One study that did examine the effect of Part D on ED visits among a general Medicare population found that, using a difference-in-differences approach, Part D enrollment reduced non-urgent ED visits but not urgent ED visits (Ayyagari et al., 2017). The authors’ findings support our conclusion in this study: Part D may have led to better health management and reduced unnecessary ED usage.
The current study excluded Medicare Advantage (MA) enrollees; they are not included in SEER-Medicare, although they represent a large and growing group of seniors. Part D studies using only Medicare FFS claims could be susceptible to confounding because of an increase in MA enrollment during the study period. We addressed this by including an index year indicator in the models, which adjusts for differences in MA enrollment over time. We have no reason to suspect that including MA enrollees would have changed our findings; however, this is an area deserving further exploration.
Although we have characterized groups in terms of complete vs. partial/no Part D enrollment, most of those in the partial/no Part D group probably had some kind of insurance coverage for prescription drugs, whether through a former employer or union, some other program, or self-funded; still, an estimated 12 percent of Medicare beneficiaries had no drug coverage during the study period (Hoadley et al., 2014). Unfortunately, our data did not provide any information about alternative coverage or lack thereof.
Our outcome measures were limited to ED visits and hospitalizations during the 12-month period after diagnosis. Despite these limitations, our study’s relatively large sample of more than 25,000 Medicare beneficiaries, along with a matched-cohort design using the reliable and well-validated SEER-Medicare database, allows us to get a clearer picture of costs, enrollment patterns, and health outcomes among people with CRC and similar people without cancer. Future research is needed to understand a broader set of potential outcomes, including OOP costs, ideally with better information on prescription drug coverage among those not covered by Part D.
Acknowledgements
The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or RTI International.
Funding support for Lisa M. Lines, Sujha Subramanian, and Sonja Hoover was provided by the Centers for Disease Control and Prevention (Contract No. 200-2008-27958-0036, to RTI International). The authors declare no conflicts of interest.

