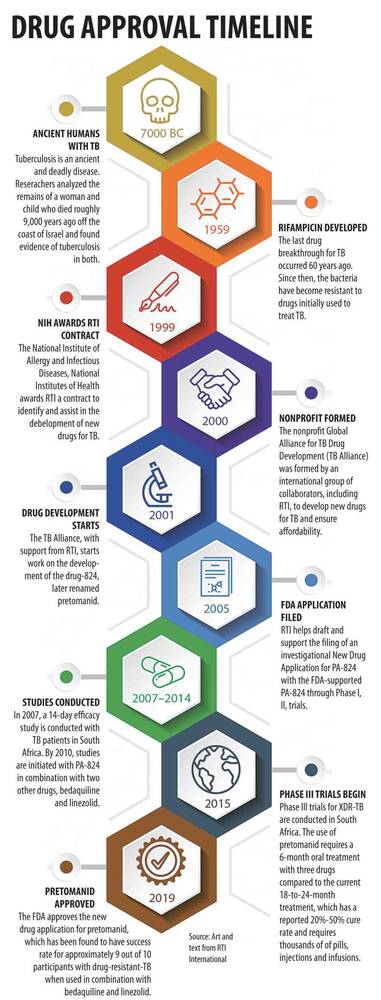
Developed by TB Alliance, a non-profit public-private partnership that RTI helped to form in 2000, pretomanid is only the third new anti-tuberculosis drug approved by the U.S. Food and Drug Administration (FDA) in more than 40 years. It has been approved by FDA as a treatment for extensively drug-resistant tuberculosis (XDR-TB), a type of TB that is resistant to two of the four main drugs that are given for TB around the world, plus any fluoroquinolone and one or more of three injectable second-line drugs.
The drug shows success for approximately 9 out of 10 participants, with a six-month treatment period when combined with two other oral drugs. Current treatment takes 18 or more months with less than half this level of success.