Improving TB treatment through drug development, industry partnership, epidemiology, and implementation
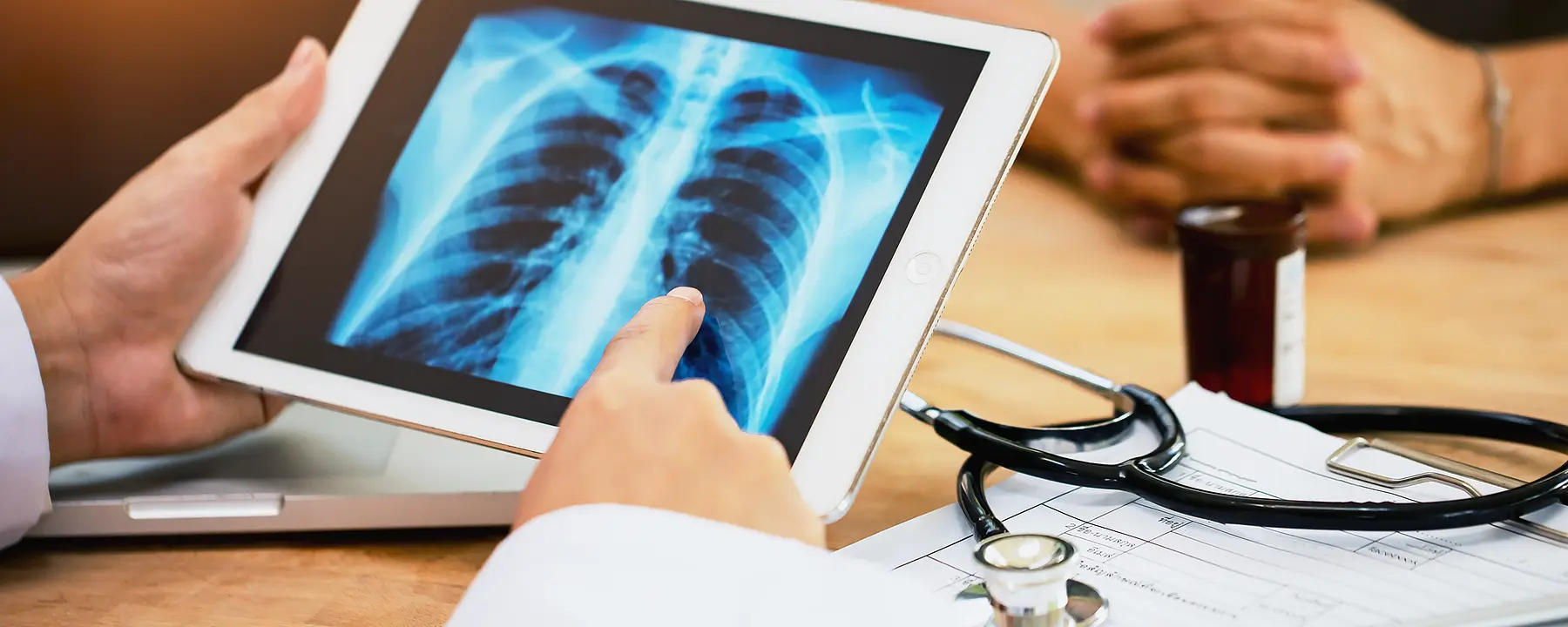
Tuberculosis (TB) is a lung disease caused by an airborne germ that is spread from person to person. Left untreated, this disease can damage the lungs (pulmonary TB) or other parts of the body (extra-pulmonary TB). TB is the leading infectious disease in the world, surpassed only by COVID-19 in 2021 as the leading cause of death from infectious diseases with 1.6 million deaths globally. Although reliable treatment for TB has been available for decades, communities around the world continue to be impacted by TB due to inequities in global access to TB commodities, shortfalls in global research and development to identify improved diagnostics and medicines, and weakness in systems to deliver TB services.
Improving access to TB treatment requires action and the necessary resources and involvement from international health organizations, philanthropic organizations, governments, and industry leaders. RTI has proven to be a leader in treating TB through its work in drug development, epidemiology, global health, and industry and public health partnership.
Tuberculosis Drug Development and Industry Partnership
RTI has over 20 years of experience developing new treatments that combat drug-resistant forms of TB, which are strains of the disease that do not respond to the primary drugs for treatment. We bring our expertise to TB treatment formulation development and preclinical and clinical studies with the goal of improving therapeutics.
RTI convened multiple partnerships with the support of the National Institute of Allergy and Infectious Diseases. In 2000, RTI formed TB Alliance, a non-profit public-private partnership, and through this partnership, RTI developed Pretomanid, a treatment for extensively drug-resistant TB (XDR-TB). In 2019, after an extensive analysis of the drug, it became the third anti-TB drug approved by the U.S. Food and Drug Administration (FDA) in over 40 years. RTI continues to be at the forefront of drug development as the disease evolves.
Tuberculosis Epidemiology
Identifying new TB infections that influence drug development, treatment, and future research are critical for combatting the disease. Through TB skin testing, tuberculosis treatment programs, and public health organization partnerships, our scientists determine local epidemiology patterns that are specific to a region. Focusing on the epidemiology of the disease allows us to formulate unique solutions to control the spread of TB in vulnerable populations.
Supporting Global Communities, Policies, and Systems
RTI supports TB diagnosis and treatment in global populations by partnering with USAID and other global leaders to address TB stigma, increasing rapid molecular testing, and increasing the response to outbreaks. We are dedicated to implementing complex multi-sector projects that align with USAID’s Global Tuberculosis Strategy and the World Health Organization’s End TB Strategy by 2030. Our multi-sectoral team is well positioned to strengthen the delivery of services to maximize the detection of TB, equip individuals to complete treatment, stop the spread of new infections, develop new innovative approaches to combat TB, and build country-owned TB systems in collaboration with local partners. Because the cost of TB related programs can lead to poverty cycles in many countries, we leverage emerging technology, medicine, and financial tools that allow for governments to plan funding streams and cost out health care programs.
Project Highlights
- TB Drug Development Support, 2005 to date (funded by the Global Alliance for TB Drug Development
- Health Policy Plus, 2015-2022 (funded by USAID)
- Tuberculosis Epidemiology, 2001-2011 (funded by the Centers for Disease Control and Prevention)
- Tuberculosis Technology Transfer Support, 1999-2011 (funded by the National Institute of Allergy and Infectious Diseases)
- Pan African Consortium for Evaluation of Antituberculosis Antibiotics, 2008, (funded by the Bill & Melinda Gates Foundation)
TB research must be innovative and meticulous to identify the best preventative and treatment programs and drug development. RTI continues to improve TB research, leveraging partnerships and science while providing TB treatment in unreached places.